|
 |
- Search
| Int J Fire Sci Eng > Volume 37(1); 2023 > Article |
|
Abstract
The present study conducted a series of fire tests using a cone calorimeter to understand the effects of pure polymers and polymer mixtures on fire properties. An initial fuel mass of 50 g in a 10-cm-diameter pan was placed on the load cell. Polystyrene, polyethylene, polymethylmethacrylate, polystyrene-polyethylene mixture, and polystyrene-polymethylmethacrylate mixture was used as fuel. The fuel ignited using a pilot burner was allowed to burn freely. The fire properties of polymer mixtures, such as the mass loss rate, the effective heat of combustion, the CO yield rate, and the soot yield rate, did not depend on the mixture composition or fuel types, suggesting it is difficult to apply the statistically representative values of fire properties of the mixture. Therefore, quantitative measurements based on experiments and their database are necessary for reasonable fire analysis.
Fire analysis models used for performance-based fire safety design or fire risk assessment require various input parameters such as realistic boundary conditions or accurate material properties, in addition to the validity of the model itself or the verification process, in order to ensure the reliability of the results [1,2]. The combustion properties of a fire source are a critical factor that directly affects the predictions of the fire analysis models, making it crucial to construct a database of the physical properties of the combustible material in fire scenarios through direct and quantitative measurements. However, in most practical fire analyses, due to the lack of material property information and the complexity of combustibles, the combustibles in the space are estimated, and values obtained from default values provided in the analysis model, literature, or related research data are often applied. However, when referring to previous studies to set the fire properties, there is a risk of errors occurring between actual phenomena and analytical results by applying nominal values of fire property in situations where there is no clear definition of combustibles or lack of material property information.
The main input parameters related to the combustible burning, which are applied to simple chemistry combustion models used in fire analysis models such as FDS, include the chemical formula of the fuel, effective heat of combustion, and yield rate of combustion products, which can be measured mainly through standard tests such as ISO-5660 cone calorimetry test or ASTM-E2058 [3,4]. Tewarson quantified the heat and the combustion products of various combustible materials and constructed the respective databases of fire properties to set the fire source of fire analysis models [5].
In most cases, real combustibles are composed of a variety of substances rather than being composed of a single-component substance. When constructing fire scenarios, it is often analyzed by applying statistical averages for materials that govern the fire characteristics in the space or by applying the maximum value of combustion properties of individual substances that make up the mixed combustibles, rather than setting the combustion properties of individual substances that make up the combustible. The fire properties of a combustible materials are influenced by combustion conditions or material combinations, which could lead to difficulties in predicting the combustion properties of a complex combustible material if the representative value of only one material in the mixture is used in the analysis. Therefore, it is necessary to understand the major changes in fire characteristics according to the mixture of individual components and to build a database based on this information in order to apply valid combustion properties for combustibles composed of various substances in fire analysis.
In this study, a series of fire test was conducted on different sample materials with relatively well-known fire properties: single polymer pellets of polystyrene (PS), polyethylene (PE), and polymethylmethacrylate (PMMA) and PS-based two-pellet mixtures. The fire properties of mixtures and the changes of fire properties according to the combination of combustible materials were quantitatively analyzed to provide database for fire properties of real combustibles.
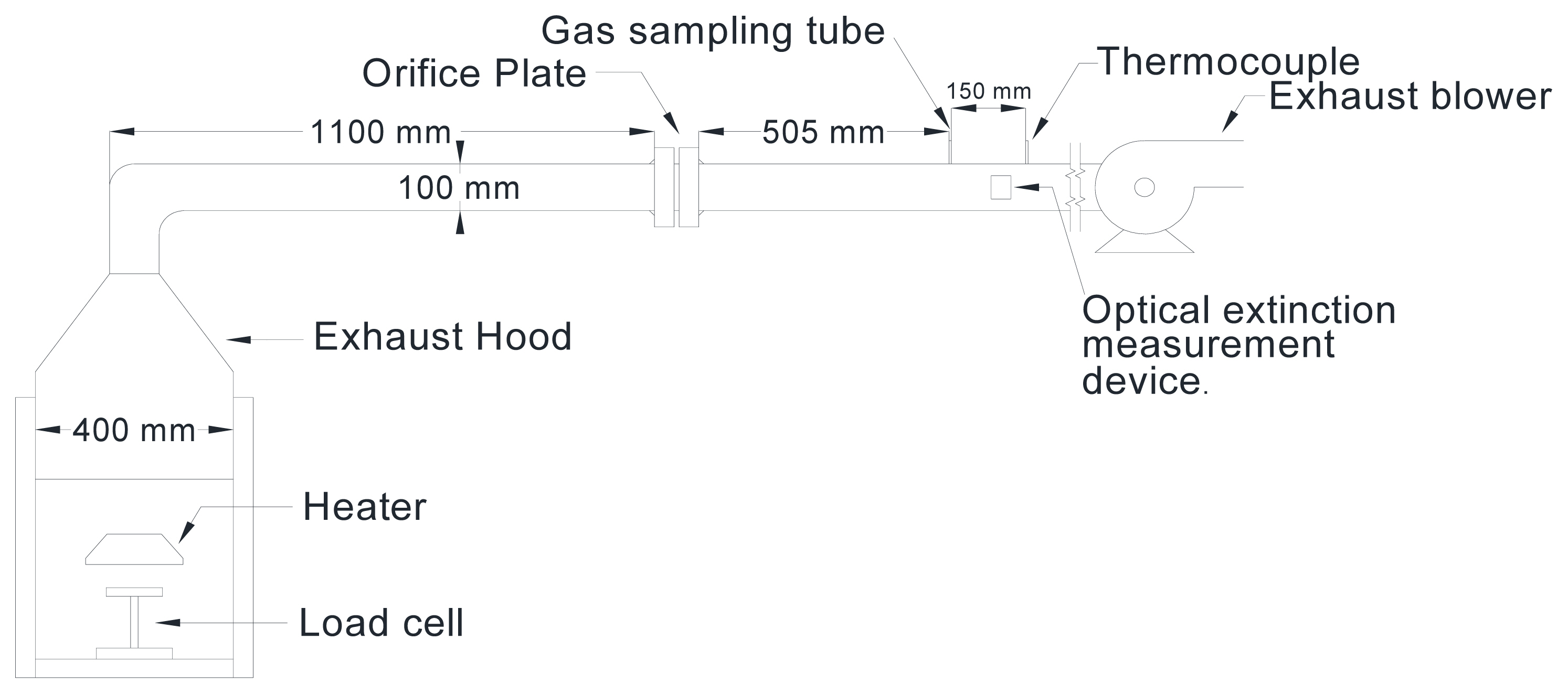
Fire tests of the pure polymers and polymer mixtures were conducted to measure their fire properties. Figure 1 shows a laboratory-scale fire calorimeter equipped with a 0.4 ├Ś 0.4 m hood and comprising a sample burning part, exhaust system, combustion gas analyzer, and DAQ(data acquisition) system. The mass changes of the combustibles during the fire test were measured using a load cell with a maximum measurement mass of 5 kg and a measurement error of 0.03%. The exhaust system is connected to the hood, duct, orifice plate for exhaust flow measurement, sampling tube for combustion gas, thermocouples, laser-based light extinction measurement device, and exhaust blower. The combustion gas analyzer comprised a gas conditioning unit to remove moisture, particulate, and coalescing liquid from the sample gas and gas analyzers. A paramagnetic oxygen analyzer (Oxymat61, SIEMENS) was used to measure the oxygen (O2) concentration [6], while a nondispersive infrared absorption (NDIR)-based gas analyzer (Ultramat23, SIEMENS) was used to measure carbon monoxide (CO) and carbon dioxide (CO2) concentrations [7]. A c-DAQ device from National Instruments (NI) was used as the data acquisition system, and processed the measured signal in real-time using the Labview program.
The heat release rate (HRR) during the sample combustion is calculated based on the oxygen consumption method using Eq. (1). The heat release (ΔHC,O2) per unit mass of O2 is 13,100 kJ kg-1 [8].
Here, m e ˙ X i O
The calibration of the calorimeter was performed using a 0.1 ├Ś 0.1 m methane burner with high-purity methane of 99.9% or higher. The measured HRR of the calibration burner was evaluated by comparing it with the HRR measured by the oxygen consumption method. The HRR via the oxygen consumption method showed < 2.5% relative error against the calibration burner, indicating adequate levels of resolution and reliability for measuring the fire properties of sample materials.
This study aims to understand the fire properties of a mixture by focusing on the effective heat of combustion and the yield rate of combustion products, which are the basic input parameters for fire analysis. The effective heat of combustion (kJ kg-1) of a material in the burning condition can be estimated based on the measured HRR and the mass loss rate of the fuel and is calculated using Eq. (3).
However, the heat release rate and mass loss rate show an unsteady characteristic during the burning process, so the difference can be shown depending on the sampling time interval or the calculation method of representative values. The mean effective heat of combustion was estimated by considering the heat release energy and mass change in the time interval to apply the mean values in this study using Eq. (4).
The yield rate (g/g) of each combustion gas, defined as the ratio of the mass of species produced during the burning of a unit mass of the fuel, is estimated based on the mass loss rate of the fuel and the mass flow rate of each combustion gas passing through the exhaust duct using Eq. (5).
Here, the mass flow rate of each combustion gas is calculated using Eq. (6) based on the concentration of the chemical species and the mass flow rate of the exhaust gas.
Soot concentration in the combustion gas was measured by the light extinction method. The mean soot yield rate is estimated using Eqs. (7-9), considering the mean soot density for the time interval.
Here, Žä is the light transmission through soot, L is the optical pathlength (m), and xm is the mass-specific extinction coefficient (1/m). The widely used value is 8700 ┬▒ 1100 m2 kg-1 at the light source with a wavelength of 632.8 nm for flaming combustion of polymer fuels [9].
In this study, the PS, PE, PMMA, PS-PE, and PS-PMMA specimens were pellet-type synthetic resins with ~5 mm mean diameter. A stainless-steel pan of 0.1 m diameter was filled with 50 g of a specimen. A pilot burner was used for ignition. PE and PMMA were mixed with PS in equal ratios to form PS-PE and PS-PMMA because PS had a high soot yield rate for a single material. Table 1 summarizes the composition of the materials used in the fire test.
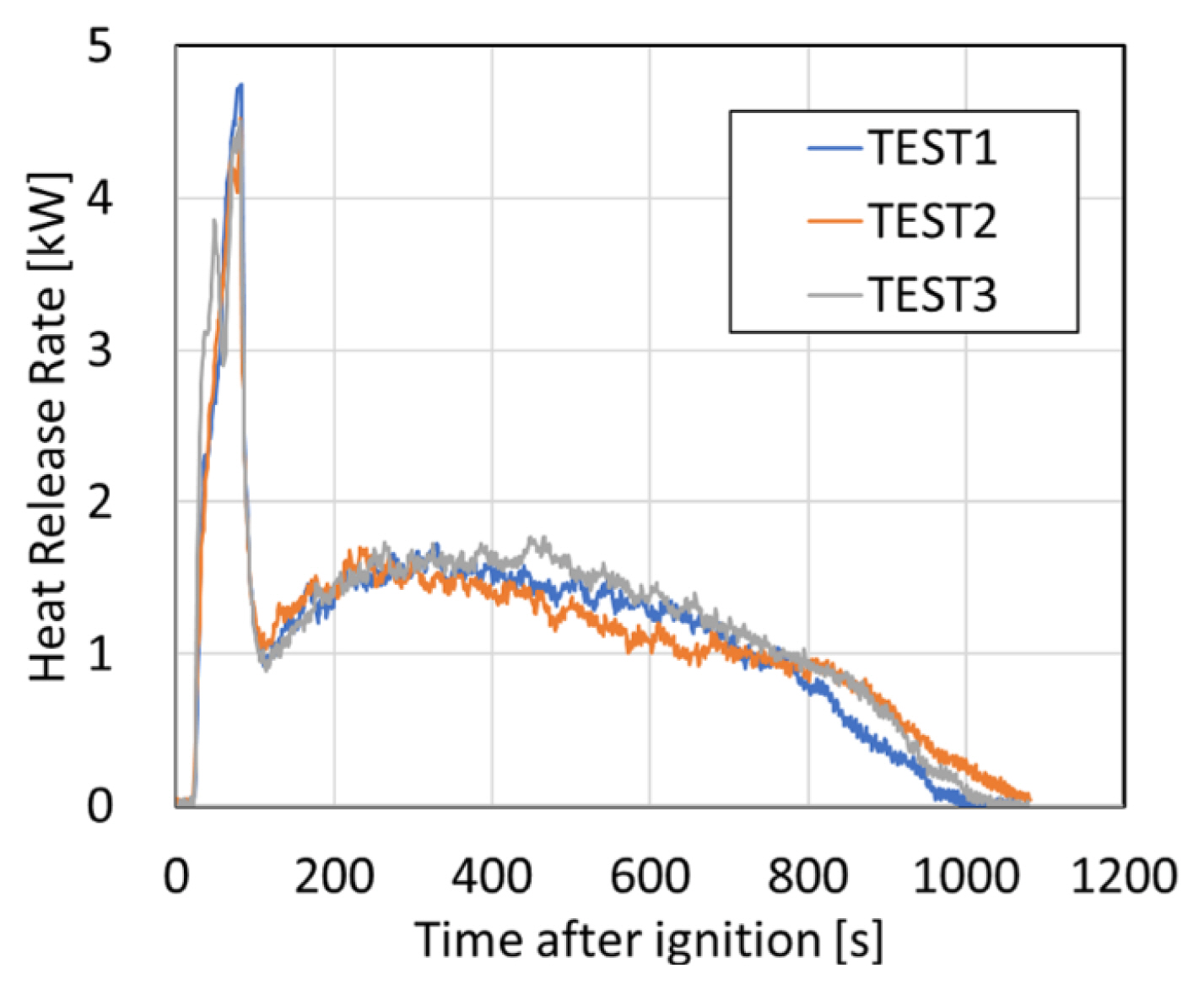
Figure 2 compares the change in the HRR of the PS pellet measured three times. Because a pilot burner was used as the ignition source, a significant amount of heat energy was applied to the fuel before ignition of the pellets, and the heat release rate increased rapidly after the initial ignition due to the ignition of the evaporated fuel during the heating process. The subsequent removal of the pilot burner causes a rapid decrease in the HRR, leading to fire growth in free burning condition. The maximum HRR < 1.7 kW at approximately 300-500 s. The HRR decreases continuously until 1100 s, with relatively high repeatability for all three measurements.
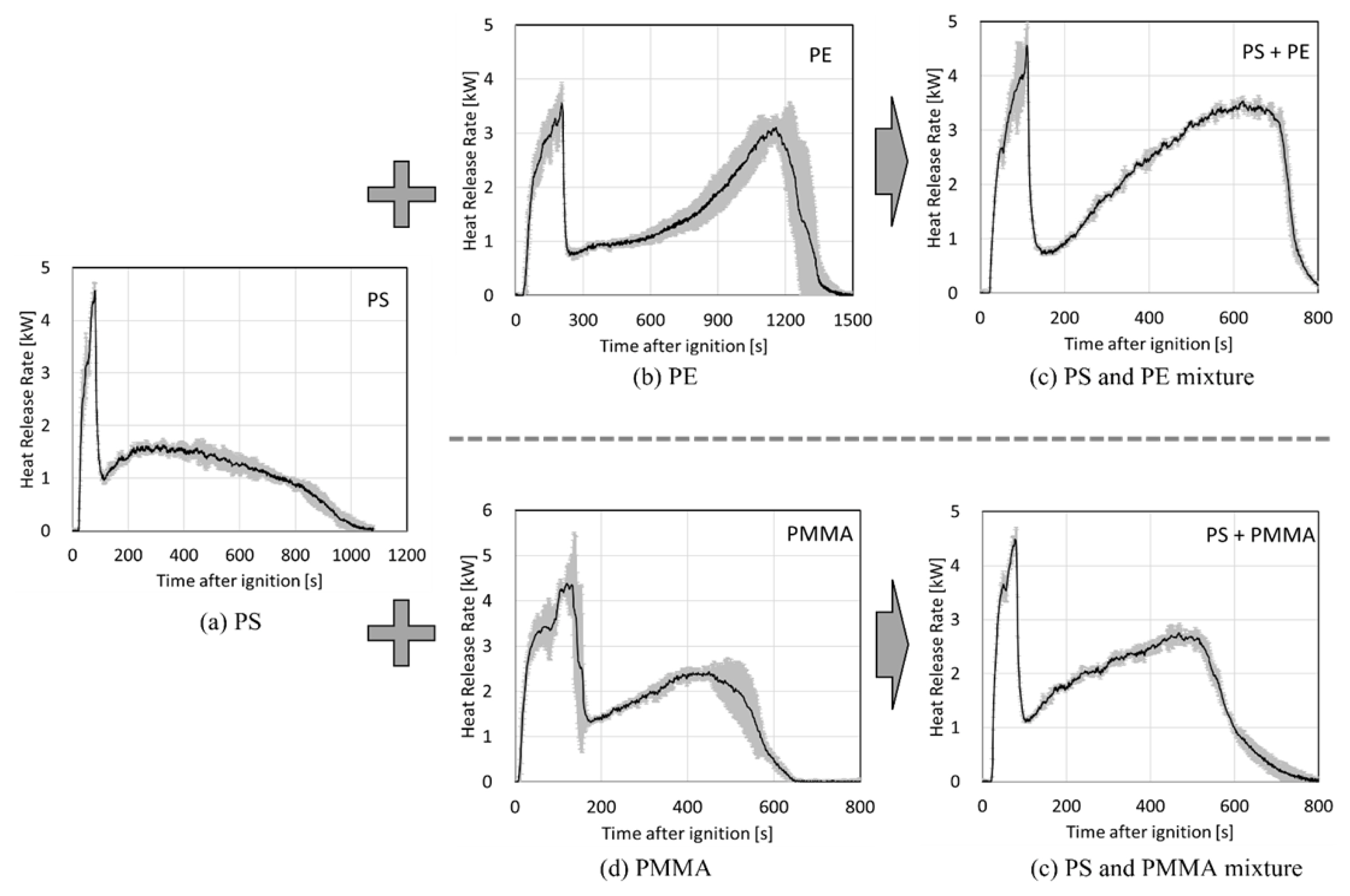
Figure 3 presents the mean HRR and the standard deviation of the three repeated measurements of the samples: pure polymers (PS, PE, and PMMA) and polymer mixtures (PS-PE and PS-PMMA). The repeatability of the test was high, except during the initial ignition stage and the fire decaying period caused by fuel depletion. The maximum HRR of PS-PE mixture is ~3.5 kW, higher than that of PE. The overall heat release rate PS-PE mixture showed a similar tendency to the shape obtained by overlapping the heat release rate curves of PS and PE. The maximum HRR of PS-PMMA mixture is ~2.8 kW, slightly higher than that of PMMA. The overall change of the HRR resembles PMMA more than that of PS.
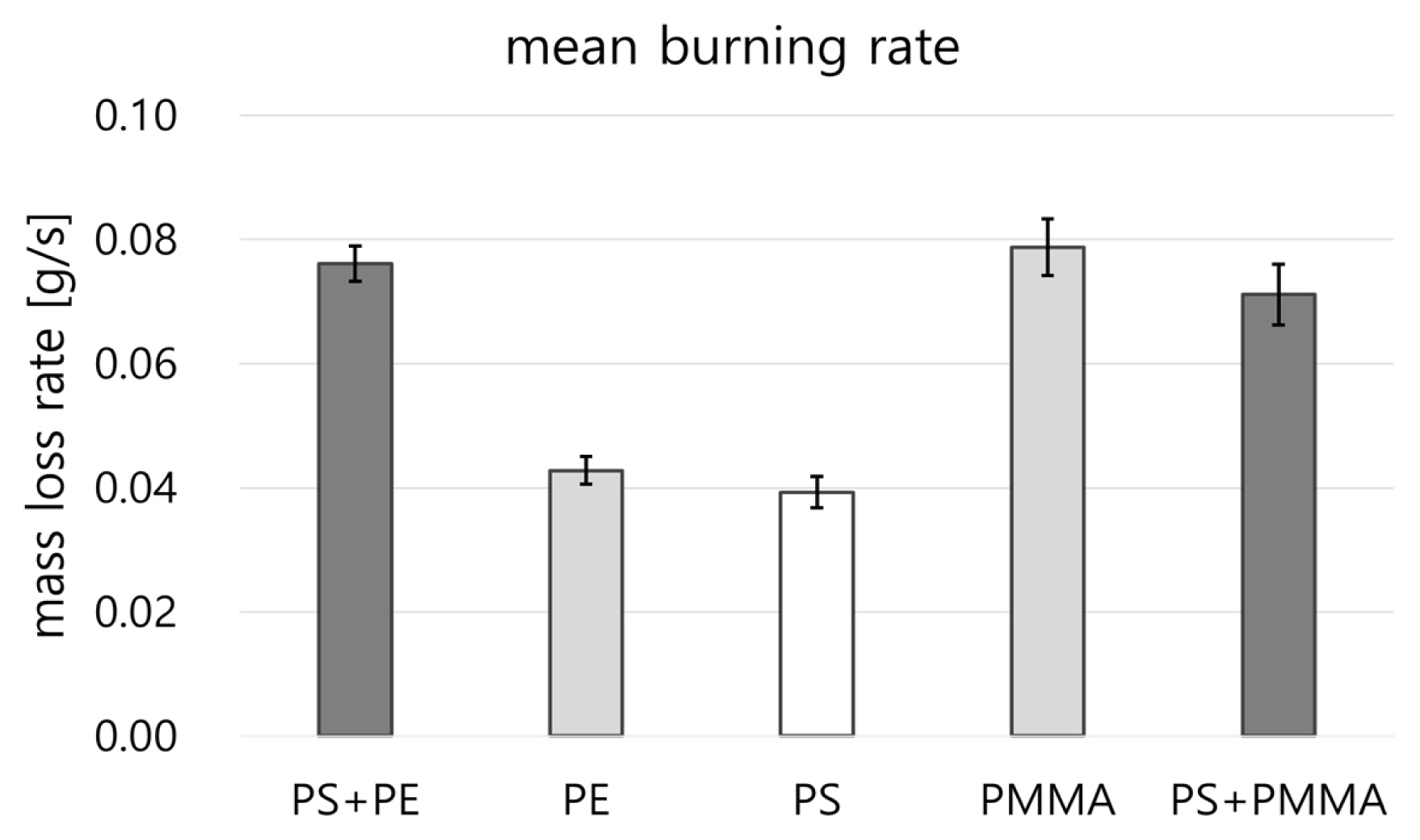
Figure 4 compares the mean mass loss rate of the combustible polymers against the mass change (Δm) during a time interval (Δt) for the three repeated fire tests. The mean mass loss rates of PS and PE are 0.04 g s-1 without any significant difference, but for the PS-PE mixture, it showed a tendency to increase by almost twice at 0.075 g/s. The mean mass loss rate of PMMA is approximately 2-fold higher than that of PS, while the mean mass loss rate of PS-PMMA mixture is close to that of PMMA.
Figure 5 compares the effective heat of combustion of the combustible polymers as calculated using Eq. (3) for the three repeated tests. The mean values of the effective heat of combustion of PE, PS, and PMMA are 42.7 kJ g-1, 35.2 kJ g-1, and 24.1 kJ g-1, respectively. The relative error is approximately 2-10% compared to the measured values in the ASTM-2058 test: 43.6 kJ g-1, 39.2 kJ g-1, and 25.2 kJ g-1 [5]. The relatively high effective heat of combustion in the ASTM-2058 test is presumed to be because the sample is heated and ignited by the radiant heater, and sufficient supply of ambient air to the sample. The effective heat of combustion of PS-PE mixture resembles PS. In contrast, the effective heat of combustion of PS-PMMA resembles PMMA.
Figure 6 compares the CO yield rate of the combustible polymer as calculated using Eq. (6) for the three repeated tests. The mean CO yield rates of the PE, PS, and PMMA are 0.0123 g/g, 0.0362 g/g, and 0.0031 g/g, respectively. The relative error is approximately 40-70% compared to the measured values in the ASTM-2058 experiment: 0.024 g/g, 0.06 g/g, and 0.01 g/g [5]. The large difference between two tests is presumed to be due to the differences in the test conditions between the cone calorimeter experiment in this study and ASTM-2058. The CO yield rate is significantly influenced by combustion or thermal conditions. The mean CO yield rates of both PS-PE and PS-PMMA mixture and PS-PMMA resemble PS with a relatively high CO yield rate. PS-PE mixture exhibits the highest CO yield rate.
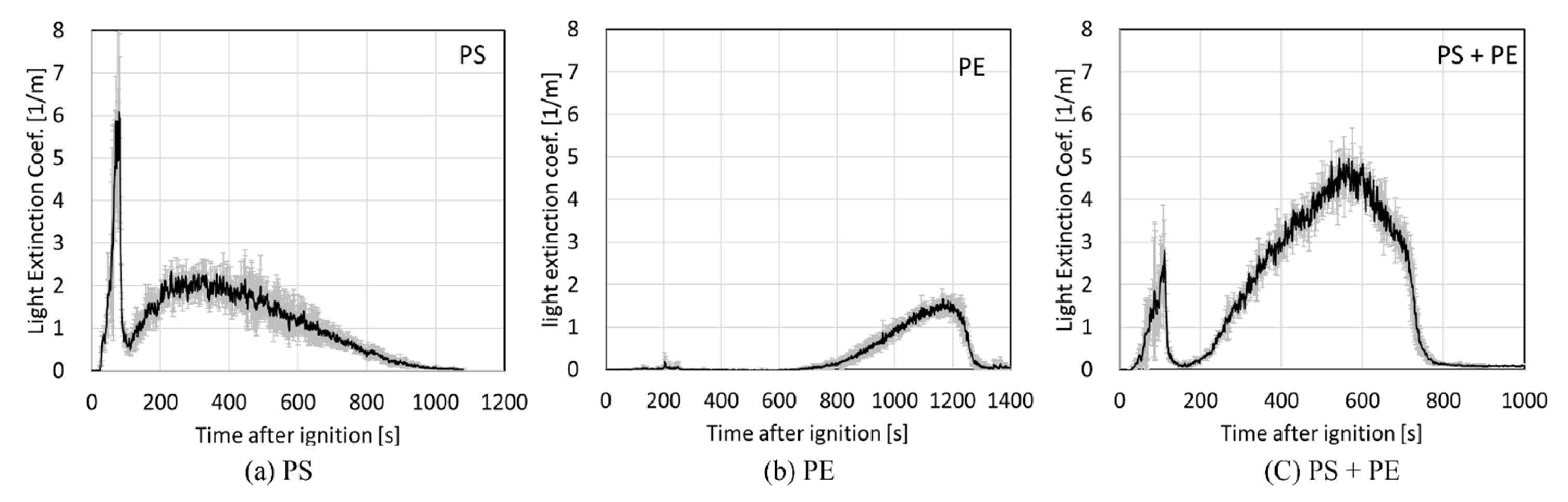
Figure 7 compares the light extinction coefficients of the three measurements at the exhaust duct upon the combustion of PS, PE, and PS-PE mixture. The graph of soot production resembles the HRR curve of PS. The soot production of PE is negligible during ignition and early combustion, but a high amount of soot production is observed in the domain of rapid-fire growth. During the initial burning processes after ignition, the smoke generation characteristics of PS were reflected in soot production of the PS-PE mixture. Additionally, it was found that the overall soot production increased.
Figure 8 compares the mean soot yield rate of the combustible polymers as calculated using Eq. (7) for the three repeated tests. The mean soot yield rates of PE, PS, and PMMA are 0.032 g/g, 0.09 g/g, and 0.0088 g/g, respectively. The relative error is approximately 45-60% compared to the measured values in the ASTM-2058 test: 0.06 g/g, 0.164 g/g, and 0.022 g/g [5]. Similar to the CO yield rate, the soot yield rate is also greatly influenced by burning and thermal conditions. It is believed that the influence of environmental differences in test conditions is relatively large. The mean soot yield rate of PS-PE mixture resembles PS and that of PS-PMMA is below the average value of PS and PMMA.
We conducted a series of fire tests on combustible pure polymers and their mixtures using a cone calorimeter to analyze the changes in fire properties according to the mixing of combustible materials. The conclusions are summarized as mentioned below.
ŌĆó The repeatability of the tests using a cone calorimeter was high. Compared to the conventional ASTM-2058, the effective heat of combustion, the CO yield rate, and the soot yield rate varied by 2-10%, 40-70%, and 45-60%, respectively, presumably attributed to the differences in combustion conditions or thermal states that caused a large variation in the yield rate of the combustion products compared to their thermophysical properties.
ŌĆó Fire properties such as mass loss rate, effective heat of combustion, CO yield rate, and soot yield rate varied significantly based on the combustible mixtures, with each mixture providing unique results without depending on a specific combustible or the mixture composition. This result suggests that it might not be appropriate to set the fire properties of the mixture constituent at the maximum values or to assume a weighted average of the mixture composition with respect to fire properties.
ŌĆó In the future, more varied types of combustibles will be investigated with additional tests to reflect the mixing ratios to quantify the changes in fire properties according to the polymer mixtures and determine the effects on fire properties.
Notes
Author Contributions
For research articles with several authors, a short paragraph specifying their contributions must be provided. The following statements should be used "Conceptualization, J. Y. Kim; methodology, S. C. Kim; formal analysis, S. G. Min; data curation, S. G. Min and J. Y. Kim; writingŌĆöoriginal draft preparation, S. G. Min; writingŌĆöreview and editing, S. C. Kim; supervision. All authors have read and agreed to the published version of the manuscript."
Acknowledgments
This work is supported by the Korea Agency for Infrastructure Technology Advancement (KAIA) grant funded by the Ministry of Land, Infrastructure and Transport (Grant RS-2022-00156237).
References
1. E Kim and N Dembsey, ŌĆ£Parameter Estimation for Comprehensive Pyrolysis Modeling Guidance and Critical ObservationsŌĆØ, Fire Technology, Vol. 51, pp. 443-477 (2015),
https://doi.org/10.1007/s10694-014-0399-0
.

2. S. Y. Mun, C. H. Hwang and S. C. Kim, ŌĆ£CO and Soot Yields of Wood Combustibles for a Kitchen Fire SimulationŌĆØ, Fire Science and Engineering, Vol. 33, No. 1, pp. 76-84 (2019),
https://doi.org/10.7731/KIFSE.2019.33.1.076
.

3. ISO 5660-1:2015, ŌĆ£Reaction to Fire Tests - Heat Release, Smoke Production and Mass Loss Rate - Part 1: Heat Release Rate and Smoke Production RateŌĆØ, International Standards Organization, Geneva, Switzerland (2015).
4. G. Marlair, J. P. Bertrand and S. Brohez, ŌĆ£Use of ASTM E 2058 Fire Propagation Apparatus for the Evaluation of Underventilated FiresŌĆØ, Fire and Materials Conference, San Francisco, US. pp. 301-313 (2001).
5. A. Tewarson, ŌĆ£Generation of Heat and Chemical Compounds in FiresŌĆØ, ŌĆ£SFPE Handbook of Fire Protection EngineeringŌĆØ, NFPA, Quincy, MA, pp. 3-82 (2002).
6. SIEMENS, OXYMAT 61 Instruction Manual - The Oxygen Analyzer for Standard Applications, (2001).
7. SIEMENS, ULTRAMAT 23 Instruction Manual - Continuous Gas Analysis, (2016).
8. R. A. Bryant and M. F. Bundy, ŌĆ£The NIST 20MW Calorimetry Measurement System for Large Fire ResearchŌĆØ, NIST TN-2077, National Institute of Standards and Technology (2019),
https://doi.org/10.6028/NIST.TN.2077
.

9. M. F. Bundy, A. Hamins, E. L. Johnsson, S. C. Kim, G. Ko and D. B. Lenhert, ŌĆ£Measurements of Heat and Combustion Products in Reduced-Scale Ventilation-Limited Compartment FiresŌĆØ, NIST TN-1483, National Institute of Standards and Technology (2007),
https://doi.org/10.6028/nist.tn.1483
.

- TOOLS
-
METRICS

-
- 0 Crossref
- 632 View
- 12 Download
- Related articles in Int J Fire Sci Eng.
-
Experimental Study on Droplet Impact Phenomena on Burning Liquid Pool Surfaces2021 August;35(4)